


|
Stubborn papers
Science at Home |
||
|
Materials
|
Compounds
|
Videos:
download2.avi (129 KB) Sec. part Pictures: 
|
|
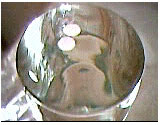
Procedure
1. With the awl make two paper circles. 2. Pour water to the glass, filling about 3/4 of the total volume of it. 3. Place the two paper circles in the center of the glass. 4. You can verify that the circles move in the glass border direction. 5. With the toothpick move them to the water surface center. 6. You can verify, again, that the circles continue to move in the border direction. 7. Remove the paper circles from the water. 8. Add more water to the glass until it has a surface above the glass limit. (the addition of the last drops should be made carefully) 9. Place the two circles in the center of the water surface. 10. You can verify that the circles don't move to the glass border. (the previous effect was canceled) 11. With the help of the toothpick move the circles for the border of the glass. (move them carefully so the water doesn't overflows) 12. After freeing them, you can observe that they move in the direction of the water surface center. (the reverse effect appeared) |
||
|
Why?
The papers have reason to be stubborn? The molecules in the surface of the water suffer strong attractions to the bulk of the liquid, not suffering up opposite attractions. This fact implicates that the forces aren't compensated and, as a result, in the water surface exists a phenomenon called superficial tension. In the particular case of the glass partially full, the center of the surface is practically plan, being the effect of the superficial tension lower. On the other hand, in the glass border exists a surface curve that is resultant of the minimization of the superficial area (surface tension). This surface curve is the zone where the superficial tension is higger. This zone is called the meniscus zone. The water, close to the paper, drag the papers in the direction of the meniscus. In contrast, for the full glass situation, with extra water, the meniscus close to the borders doesn't exists. The superficial tension is larger in the center. Therefore, the paper is dragged by the water to the center of the glass. Now you can see that the papers don't have blame to be stubborn. The blame is of the superficial tension!
|
||
No part of this website can be reproduced without previous authorization. Please inform me if there is any problem with the website. |